When blood flow to an organ is disrupted (ischaemia), such as during heart attack, stroke or organ transplantation, rapid restoration of blood flow (reperfusion) to the ischaemic tissue is essential to minimise cell death. However, even though reperfusion is essential to salvage cells, the restoration of blood flow paradoxically causes further tissue injury. This ischaemia-reperfusion (IR) injury greatly exacerbates tissue damage and therapeutic approaches to minimise IR injury are urgently required. We aim to understand the pathophysiology of IR injury in a range of clinically relevant models. By uncovering the mechanisms of damage involved, we have developed rationale approaches to target mitochondria, thereby preventing IR injury in vivo.
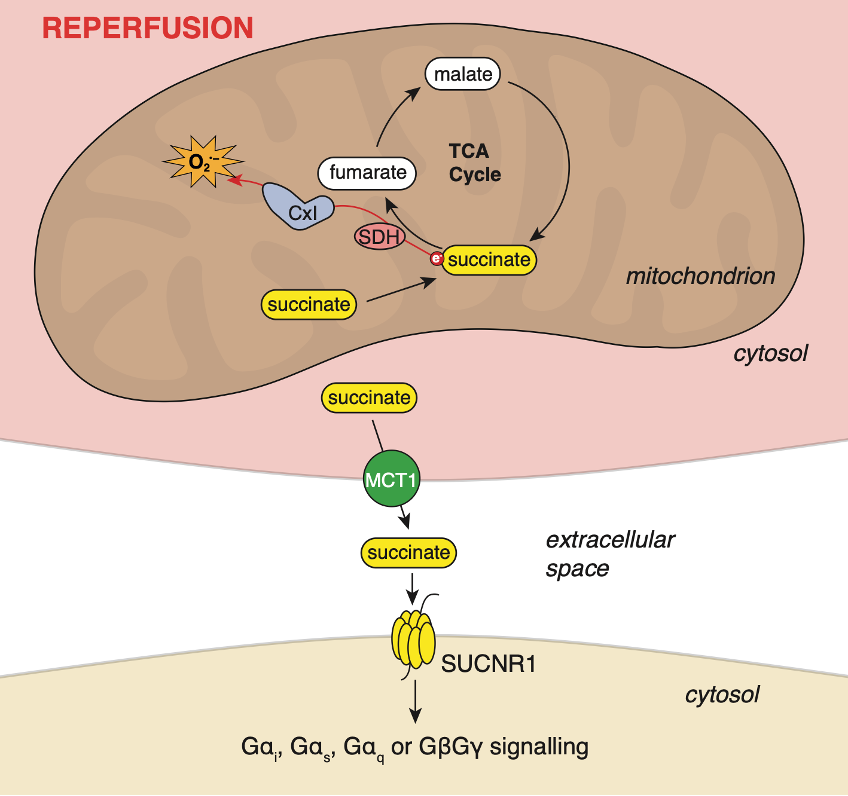
Mitochondria are more than just powerhouses of the cell. They are key synthesisers of signalling molecules, capable of communicating between cellular compartments. Much of our knowledge of mitochondrial signalling is limited to energetic and redox balance, and a particular focus on reactive oxygen species as intracellular signals. Recently, however, the idea that mitochondrial metabolites, derived from the tricarboxylic acid (TCA) cycle, can act as signalling molecules is gaining traction. While the transport of mitochondrial metabolites from the mitochondria to other intracellular compartments is well defined, the intercellular signalling of these molecules is poorly understood. Therefore, our knowledge of the mechanisms by which mitochondrial metabolites can exit or enter cells and how these processes may act to influence cellular function may help to understand their role in diseases.
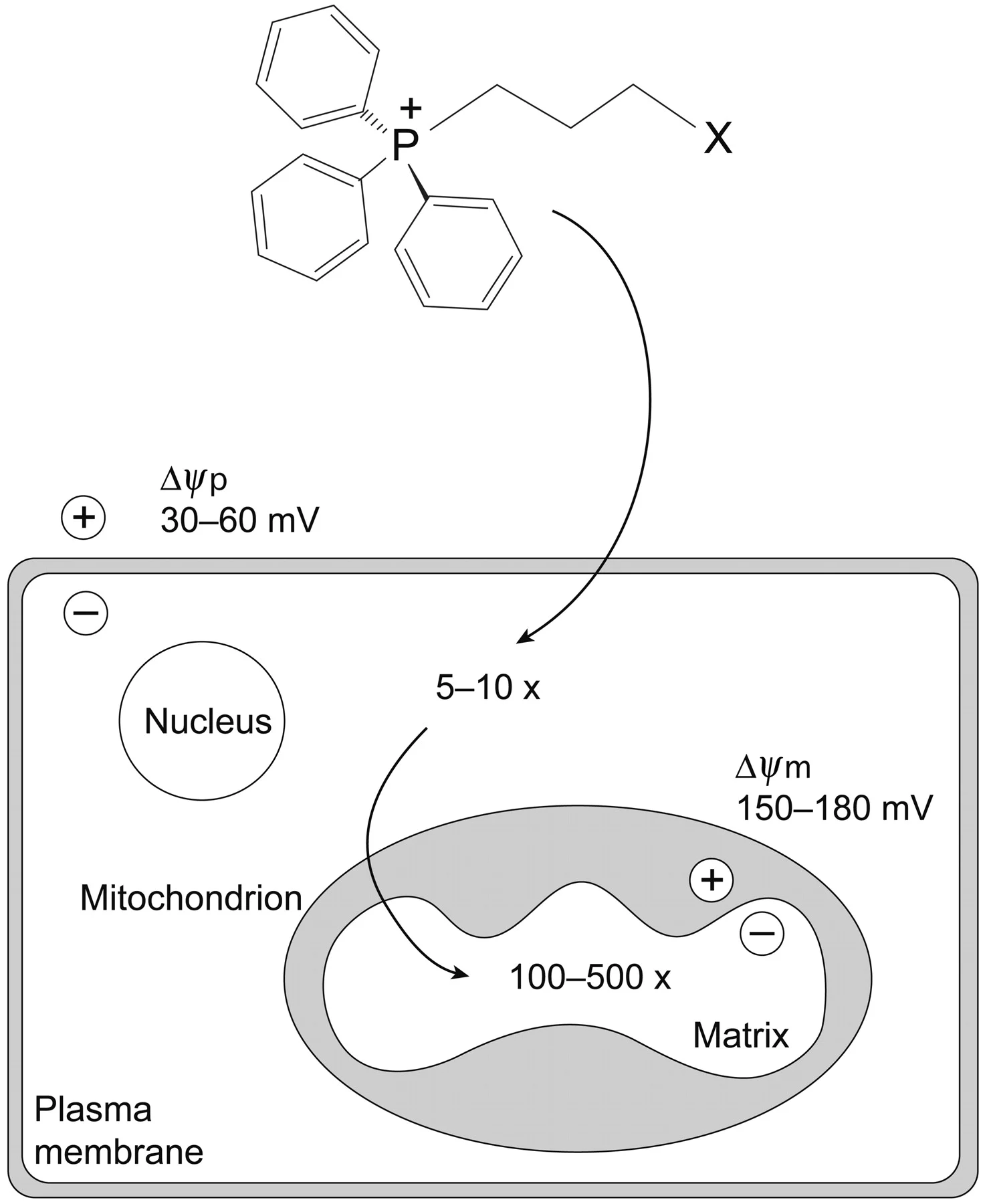
There is considerable interest in targeting molecules selectively to mitochondria. A common approach is by the use of the lipophilic triphenylphosphonium (TPP) cation, which is membrane permeable and drives the selective mitochondrial accumulation of attached moieties in cells and in vivo. TPP cations achieve >100–1000-fold accumulation within mitochondria due to their mitochondrial membrane potential dependent uptake. This approach has been used extensively to target a range of probe and drug molecules to mitochondria in vivo, providing both new insights into mitochondrial biology and potential therapies. We aim to use mitochondria-targeted approaches to develop new probes and therapies to better understand the role of mitochondria in diseases and to develop selective, targeted therapies.